
If you would like to submit a written tribute in honor of Professor Stewart E. Novick, please e-mail chemistry@wesleyan.edu.
Susanne Fusso
Colleague and friend
When I came to Wesleyan in 1985, it wasn’t easy to make friends outside my department. Happily, Annie Dillard, who presided over the “round table” in the Faculty Club in Downey House, had a talent for gathering not only literary, artistic, and social-science types, but also the wittiest and most amusing scientists on the faculty to have lunch together. Without the round table, I don’t know that I would have come to know Spencer Berry, Jack MacIntosh, David Westmoreland, and other amazing thinkers from the third division. Among them, Stew Novick had a special place in my heart. Stew’s combination of brilliance, humor, acerbity, and kindness are a rare and potent mix. I felt a bond with Stew through his love of cats and exasperation with committee service, but most of all I always felt his warmth — for his students, his colleagues, and above all his wife Judy, whom he always spoke of with deep affection and acknowledged as an authority, even though there were few authorities to whom he would bow. It is very hard to realize that I will never again have lunch with Stew. I am so grateful to have known him, and I will always remember him with love.
Ellen Anderson
Wesleyan PhD, colleague, and friend
I was a graduate student when Stew Novick arrived at Wesleyan in 1978. Initially, I was in a lab down the hall but I soon moved to the lab next door to Stew’s office. In 1980, my daughter was born and she spent a lot of her first year of life in my lab. Although she was often sleeping or playing in her playpen or out for a walk with me, there were times that she was not quiet, to say the least. Stew was such a good sport about the situation; he put up with the noise and never once complained to me. After I defended my Ph.D., I have come back to visit the department frequently over the past 41 years. My first stop was often Stew’s office. He was always happy to see me and we would catch up on what was going on in our lives. I am honored to count him as one of my longtime friends and I was so saddened to hear of his death. He was a superb teacher, a brilliant researcher and a consummate gentleman. You will be sorely missed, Stew.
Ellen Anderson, Ph.D. 1982
Associate Professor, Retired
Department of Chemistry
University of Saint Joseph
Daniel Frohman
Wesleyan PhD (former student)
Some of my favorite memories of time shared with Stew are those in which we shared hobbies. These include: Stew collected coins amongst other things. I also have long shared this hobby and brought in my collection to show him. We both were excited about astronomy and would discuss meteor showers or any notable space-related news. Stew introduced me to the work of a favorite author of his, Terry Pratchett. This quickly became my favorite author, and I over the years picked up books and would loan them to Stew to read when he hadn’t read a particular one. I also fondly remember various times we got together to share a meal. Sometimes this was a group gathering. Other times were smaller gatherings, such as when we were stranded in D.C. while going to a conference, and we went to a sushi place and then walked after dinner to see the Washington Monument.
Jennifer van Wijngaarden
Colleague
I first met Stew when I was a graduate student and attending my first academic conference at Ohio State University in 1998. Although my talk was scheduled for the very last day, Stew was in the audience and even asked me a question.
Although I met Stew annually at that same meeting (even after it moved to Illinois), I formed a closer scientific connection in 2004 when I accepted a Visiting Assistant Professor position for Mount Holyoke College. I reached out to Stew and asked if I could be involved in any ongoing research projects to give me a bit of an outlet as I was transitioning into my first academic appointment with a high teaching load which felt a bit isolating at times. Stew’s response was immediate and enthusiastic. I visited Wesleyan soon after and he handed me a key to his lab and invited me to come as often as I liked. I generally visited about once per week for a full day during the period 2004-2006 and worked on a few different projects with Stew’s graduate students and Pete Pringle during this time. I was also welcomed into Stew and Judy’s home to meet their cats and have dinner and in later years, we would always swap stories about our cats when we met.
In 2006, I accepted a tenure-track appointment at the University of Manitoba in Winnipeg, Canada with the plan to establish my own research group in microwave spectroscopy. With limited resources to build a custom spectrometer (about $70k USD), Stew gifted me a few relics from his own collection and connected me with a colleague who’d recently retired from Rensselaer (Woody Gillies) and his partner (Jennifer Gillies) at Siena College who was leaving academia. With Stew’s introduction, I was able to collect about 90% of what I needed to build my first FT microwave spectrometer to move to Winnipeg with me in July 2006. One complication was that some of the equipment had been donated to Jennifer from NIST and while the U.S. government had deemed it surplus and didn’t want it back in Maryland, they didn’t want it going to Canada either. Stew placed many phone calls and emails on my behalf to colleagues at NIST including Jon Hougen and Gerry Fraser and it was decided that the equipment could be transferred to Stew officially and then beyond that, NIST was not interested in what happened to it. Although Stew often joked that he didn’t want to go to jail if they came looking for this equipment, the matter seemed to have been forgotten with time. Now, 18 years later, I still have that spectrometer running and generations of my undergraduate and graduate students have used it in their research. I moved to York University in 2022 to become Chair of Chemistry and have now re-assembled this well-travelled instrument in Toronto where my current students are working away most days with it.
In my early years as a faculty member, I would call up Stew for advice every few months about something I didn’t understand (e.g., Pickett’s program, complex tunneling problems, etc.) or to point me to someone who could repair something in my lab including Jens Grabow from Hannover, Germany. Stew always seemed to be ‘in the know’ and lead me in the right direction and for that, I am truly grateful. It is so difficult to believe that after 25 years, I cannot call up Stew and ask for his guidance. He was instrumental in my career progression and research success. Always incredibly supportive, kind and patient. He was an excellent role model for how we should all treat our students and colleagues and I will truly miss him.
Jennifer van Wijngaarden
Chair, Professor, York University (Toronto, Canada)

John Keith
Wesleyan alumni (former student)
Stew was one of my many favorite professors at Wesleyan who were in the chemistry department. I still recall my 2nd semester chemistry class where he challenged students to point to every element of the periodic table in time with Tom Lehrer’s “Elements” song. Stew somehow always managed to always balance his passion for chemistry, explaining key concepts clearly that had been muddled in my head until that moment, while also being self-disciplined as an excellent lecturer (the image of him fluidly turning off his watch alarm that started beeping two minutes before the end of lecture, not matter what he was doing at the time, always stands out to me).
At the end of my senior year, at a time when I was probably beaming with unbridled confidence, he was the only faculty member that I recall who made a point to return his comments to my undergraduate thesis in person. He handed the hardcover book to me, said he enjoyed reading it, but then he immediately opened to a page showing a figure depicting solutions to the harmonic oscillator problem that I had created. He stated that while I drew the energy differences correctly as h-bar omega, he was confused why the ground state solution drawn to be 1/2 h-bar omega as we learned in class (tacitly, in two of the classes I had taken from him). In hindsight, it was the perfect learning moment at the end of my college, and it was just one of the many subtle, precise, perfectly-timed, and wholly appropriate comments he made over the years I worked in Hall-Atwater. I am very glad I had the privilege of having classes from him.
Peter W. Park
Wesleyan alumni ’93 (former student)
Stewart Novick was my General Chem and Physical Chem professor, but he also served as my unofficial advisor for years. He had a profound influence on my career and time in the department. His love of science, commitment to scholarship, and sense of humor made me want to be a scientist. When I got accepted to Princeton but rejected from a certain school in Cambridge for my PhD studies, he actually cussed them out! Jokingly, of course. I think. I can honestly say I would not have pursued a career in science were it not for Stew and Pete Pringle. Every college student needs a faculty member like Stew in their lives to seriously guide them while showing them that work should be fun.
Carol Sherwin
Wesleyan alumni (former student)
Prof. Novick was a warm and caring professor. I remember when i first met him in 1987; he was funny, lighthearted, and totally committed to his research work and had time to talk with me whenever i came to see him. His impish smile sits before me now, and I recall how much he loved his wife (i think he got married while i was a student on campus). His unswerving encouragement of me as a female chemistry major still lives in my memory, and i will always be grateful to him for the countless hours that he spent inside and outside the classroom helping me understanding both theory and complex processes. May he remain always in our hearts.
Daniel McCamant
Wesleyan alumni ’95 (former student)
Stew Novick was the person that got me interested in Quantum Mechanics, which later became what I built an academic career around. It was his Physical Chemistry class and my conversations with Stew in his office after nearly every lecture that got me really interested in this area of science. I will forever be thankful that I got to take those courses with him; I learned so much and he built such an extraordinary sense of community among me and my classmates. I still keep the notes from that course on my bookshelf for when I need to remember how something is *really* derived! (See photo of my 31 year old notebook, attached.) I certainly benefitted from the kindness and camaraderie that he consistently showed to all of his students.
Later, I was able to work with Pete Pringle in an exciting new research project using Stew’s FT microwave machine. That project studying van der Waals complexes was a fascinating introduction for me, where I learned how spectroscopy can reveal the hidden behavior of the tiniest molecules. It exemplified Stew’s advice to me, that “If you like quantum mechanics and you want to work with your hands, then you should become a spectroscopist. Spectroscopy is experimental quantum mechanics!” I often repeat that phrase to my own students and hope that they can benefit from Stew’s wisdom.
I’m so sorry that I cannot attend the memorial in person, but Stew would approve my excuse: I have to teach a spectroscopy class at 9 am on Monday.

Russell Smilgys
Wesleyan alumni ’82 (former student)
I was an undergraduate member of Stew’s group in the early 1980s. I joined because I thought “the big mean molecular beam machine” was a marvel. I aspired to learn how to operate such an amazing scientific instrument. Alone in the lab, I’d walk up and down the aisle tracing the cables and looking in the ports. I had a lot to learn.
One afternoon I was in the lab at the bench playing with an oscilloscope. I connected a function generator to the scope using a coaxial cable I got off the cable rack. Stew came by and offered to give me a lesson in scope operation, which I accepted. Afterward he attempted to remove the cable from the scope, but the connector was stuck. He said that was strange. I agreed and showed him how I had solved the problem using a pair of pliers. As it happened, the cable I selected was MHV, not BNC, so it did not fit. Without showing any upset that I had stupidly forced the wrong connector, he patiently gave me a lesson in coaxial cables.
I have fond memories of working in the lab under Stew’s mentorship, and in the class he taught in quantum mechanics. Stew was the best science teacher I had at Wesleyan. After doing enough science to generate a publication, he assured me I was a solid candidate for graduate school, and he could guarantee my admission to Harvard’s program. He also raved about his time as a postdoc at the University of Colorado in Boulder. Ultimately that is where I went for my PhD in chemical-physics. Thank you Stew.
Russell Smilgys
Class of 1982
Ken Leopold
Microwave Colleague
Stew was never one to complain, even when others might have. Many years ago, we wrote a chapter with Bill Klemperer that went into a book edited by Elliot Bernstein. Stew came out to Minnesota for a few days to work on it with me. Unfortunately, being Minnesota in the middle of winter, it was 14 below zero (that’s around -26 deg C) and Stew was not dressed properly for it. But he was a real trooper and never complained. A few years later (30 years ago, to be exact), Stew and I wrote a Chem. Rev. article with Bill and Jerry Fraser. These articles have little bio-blurbs, and I collected them for everyone and sent them to the journal. This was back when we had secretaries, and mine accidentally wrote his name under the blurb as Steward, but I failed to catch it. So it got published that way. I remember sitting outside of Independence Hall at Columbus that year and apologizing profusely. Stew assured me that he wasn’t too concerned, noting that his mother was probably the only one that would care.
Stew had a way with words. The clarity and down-to-earth language in his talks always stood out to me. There were a few particularly memorable things, too. The first time I ever heard the expression “we’ll burn that bridge when we come to it” was from Stewart, and I always think of him when I use it. I also remember his comment on molecular sources. Referring to the hit-or-miss aspect of getting one to produce the species you want, he described molecular sources as “just sorcery”, which was both true and a perfect play on words.
An excellent scientist and a fine human being.
Robert Bohn
Scientific Collaborator
In the early 1990’s, Stew invited me to visit Wesleyan and present a seminar on my molecular structure studies. I am not sure if he and I had met before this event but I certainly hardly knew him then. I drove to Middletown and found Stew in his office. This was his greeting. “LET’S WRITE A PROPOSAL’. Stew did not waste any time on routine greetings.
We wrote it. In the 70’s and 80’s I had changed my research interests from electron diffraction determinations of molecular structure to microwave studies using the Hewlett-Packard Stark modulated spectrometer to identify the conformations in nearly prolate symmetric tops. Nature made a lot of them.
We invited Karen Peterson, then at the University of Rhode Island, and Mark Marshall of Amherst College to join us, called ourselves the Southern New England Microwave Consortium, and were awarded a major grant which Stewart used to build the Pulsed Jet Microwave Spectrometer which was the heart of Stewart’s laboratory for many years. That instrument joined by a chirped pulse spectrometer contributed by Steve Cooke established Stewart’s lab as a leading laboratory of microwave spectroscopy.
Robert Bohn
Professor of Chemistry, Emeritus
University of Connecticut
Karen Peterson
Colleague
Stew was a wonderful colleague and friend, not just to me, but to many people. His impact on those around him was enormous. I had known him since my graduate days in Colorado, and we were ultimately connected through a love of microwave spectroscopy. For many summers, he graciously welcomed me to conduct experiments in his lab using his FFT spectrometer. This was particularly important as I transitioned from the East to the West coast.
Stew conducted his research lab in a purposeful way, giving high priority to keeping the equipment running (not an easy feat), and giving even higher priority to his students. He prided himself in always being available to discuss projects. With regard to this, I remember him saying that he modeled his leadership style after Star Trek’s Captain Picard, who always immediately put down anything he was doing if any of his crew needed his attention. But he also modeled his style after his graduate advisor, Bill Klemperer, for whom he had the greatest admiration.
I will always remember Stew as someone who was generous with his time. He set an example for all those around him. I am very sorry he left us so soon.
Barney Ellison
University of Colorado
Stewart & Me — Almost 50 years of Friendship and Chemistry
Stewart and I were postdoctoral students together in the 1970s. In 1975 I left Yale and went to join Bill Reinhardt’s theoretical group at the Joint Institute of Laboratory Astrophysics (now just JILA) in Boulder, CO. Soon I was doing experiments in Carl Lineberger’s lab and Novick came out to Carl’s group in 1976 from Harvard.
JILA was a magical place then and it was one of the world’s premiere research institutes in Atomic and Molecular Physics. The JILA Fellows at that time were a team of front-line physicists: (Jan Hall, Jinx Cooper, Gordon Dunn, Alan Gallagher, Earl Beaty, Art Phelps, Sid Geltman, Judah Levine, Peter Bender, …). When joined with the combined chemical groups of Lineberger, Reinhardt, and Leone, this was a unique place in the world. At that time, there was no “laser store” to help you out. Jan Hall and Judah Levine designed and built their own AR II laser. (Now you just buy one for about $100k.) All of the experimental hardware was conceived and fabricated in the JILA instrument shops.
In Carl’s JILA labs, Stewart, Paul Engelking, and I studied the photoelectron spectra of negative ions:
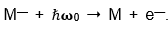
For some of the ion-beams that we photodetached,
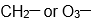
we had to use explosive samples. But no one had the laser technology that we had. And we knew what to look for and tried all sorts of anions that no one has ever seen. We just turned up the current & looked to see what happened. These were hard experiments and it was very easy to make a mistake. Stewart and I became
very close friends while struggling with these machines. We bonded even though I am an organic chemist and Novick was a real spectroscopist.
Stewart was a natural fit for JILA because he came from Bill Klemperer’s Group at Harvard. Klemperer was a pioneer in the study in interstellar molecules. Once you have a proper microwave spectrum, you know where to look in a radio telescope to identify interstellar molecules. Here is Klemperer’s group about 1974. Stewart comes from royalty.

Kit Bowen, John Winn, Richard Cavanagh, Ken Janda Stewart Novick, John Hemminger, Stephen Harris, Kelly Chance
You might think of Stewart as an Ivy-League intellectual but he was more athletic that you might guess. Here is a photo of Novick as a young guy.

(Harvard or JILA – I can’t remember)
Stewart actually taught me to rock climb when we were not in the lab. Here is a photo he took of me as the two of us when we were scaling the flatiron rock formations that are adjacent to Boulder.

JILA and the Univ. Colorado are several thousand ft. below me
In 1978 Stewart joined the Chemistry faculty at Wesleyan University and it was a perfect fit. He built a productive microwave laboratory in Middletown and was always regular at the International Symposium on Molecular Spectroscopy. Besides Stewart, I have always been a close friend of George Petersson since we overlapped at Yale. In his time at Wesleyan, George developed his own variant of molecular quantum mechanics
that he called Complete Basis Set methods. I now go to engineering conferences and see several thousand engineers modeling their large ensembles of radicals with George’s CBS-QB3 methods. Barney was an undergraduate at Trinity College in Hartford. But my college simply could never compare with Wesleyan. With Wesleyan’s faculty including scholars such as Stewart and George, they were doing research of the highest quality. To have undergraduate students exposed to basic research of the highest caliber is a unique asset of Wesleyan Univ. And Stewart was one of the guys who made it go.
Stewart has always been connected to the top circles of Chemical Physics. Here he is at the JILA Symposium, “Forty Years of Ion Chemistry.”

Stewart E. Novick, Joshua Boger University Professor of the Sciences and Mathematics
Stephen R. Leone
John R. Thomas Endowed Chair of Physical Chemistry

Eric Herbst
Commonwealth Professor: Depts of Chemistry, Astronomy, and Physics University of Virginia
W. Carl Lineberger
E. U. Condon Professor of Chemistry JILA, University of Colorado
Stewart E. Novick,
Joshua Boger University Professor of the Sciences and Mathematics Wesleyan University
Stewart had a wonderful career at Wesleyan. He became Wesleyan’s Joshua Boger University Professor of the Sciences and Mathematics and has made an enormous impact on the Sciences at Wesleyan. He was an outstanding teacher.
The last time I saw Stewart was in 2020. Sally & I drove up to Harford to spend the day. Here’s what we looked like.

Hartford, CT 2 April, 2022
As Novick and I talked at this Korean Café and he told me he had a paper in press in Journal of Molecular Spectroscopy on the microwave spectra of the
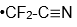
radical. (J. Mol. Spectrosc. 2022, 385, 111618.) Of course I was interested because we measured photoelectron spectra of the
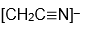
anion and this gave us properties of the
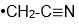
radical.) When I started to talk with Stewart about comparisons of his radical
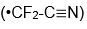
and mine
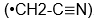
, my Sally kicked me in the leg.
One of the most important people in Stewart’s life was Judy, his wife.

JILA, 2009
Stewart was one of us. He was part of my youth.
And we were Lions then.
















